The National Environmental Policy Act of 1969 (NEPA) requires all Federal agencies to assess the environmental impact of their actions and to ensure that the interested and affected public is informed of the environmental analyses. The Food and Drug Administration (FDA) considers the environmental impacts of its actions as an integral part of its regulatory process. FDA regulations at 21 CFR Part 25 (“Environmental Impact Considerations”) specify that environmental assessments (EAs) must be submitted as part of certain new drug applications (NDAs), abbreviated new drug applications (ANDAs), applications for marketing approval of a biologic product, supplements to such applications, investigational new dr…
The National Environmental Policy Act of 1969 (NEPA) requires all Federal agencies to assess the environmental impact of their actions and to ensure that the interested and affected public is informed of the environmental analyses. The Food and Drug Administration (FDA) considers the environmental impacts of its actions as an integral part of its regulatory process. FDA regulations at 21 CFR Part 25 (“Environmental Impact Considerations”) specify that environmental assessments (EAs) must be submitted as part of certain new drug applications (NDAs), abbreviated new drug applications (ANDAs), applications for marketing approval of a biologic product, supplements to such applications, investigational new drug applications (INDs) and for various other actions, unless the action qualifies for a categorical exclusion.
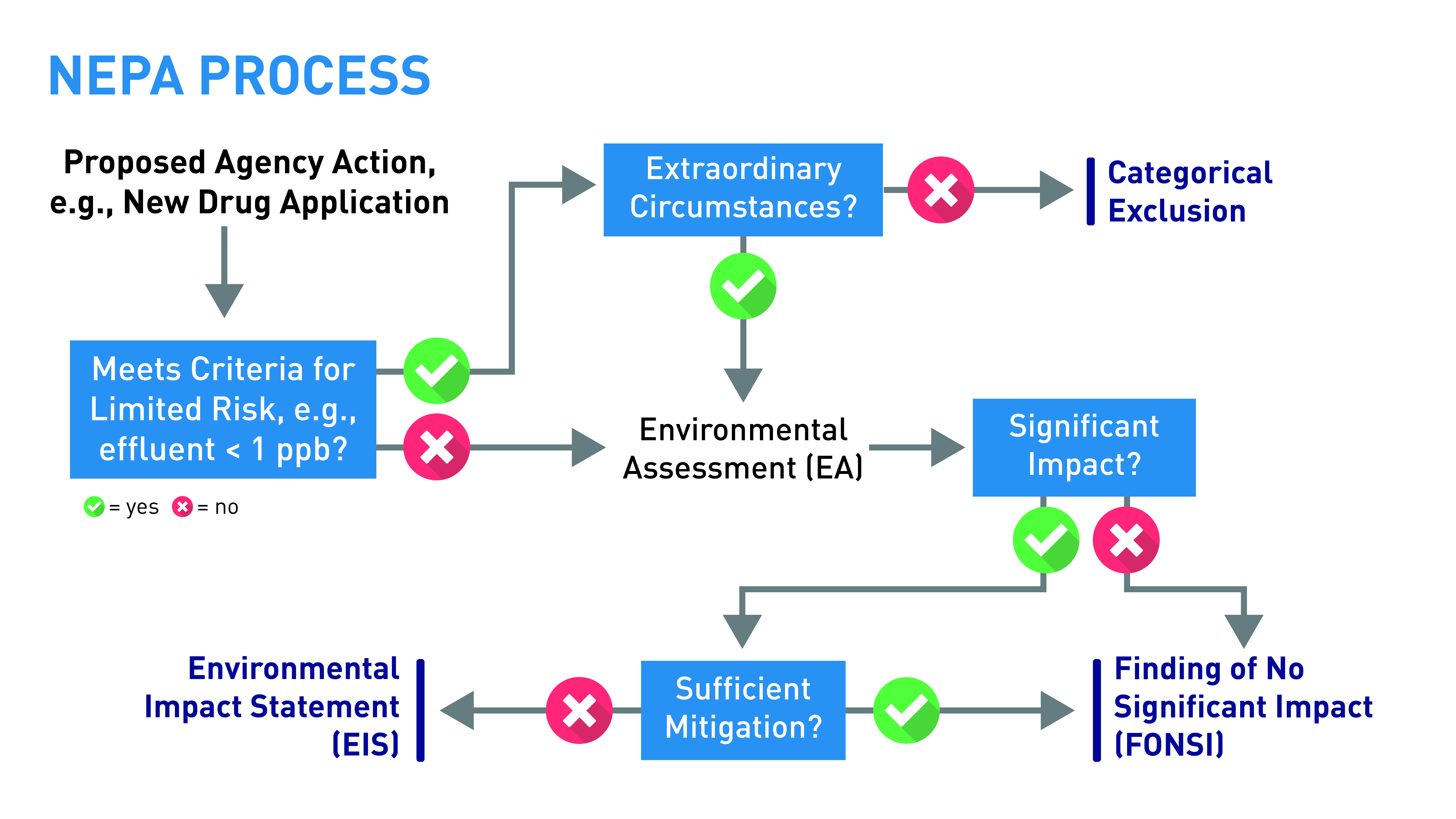
Figure 1. NEPA Review Process at CDER
FDA’s environmental impact regulations were established in 1977. Under the President’s reinventing government initiatives, announced in April 1995, FDA reevaluated and revised its environmental regulations to reduce the type of actions that require an EA and, consequently, the number of submissions (or applications) that require an environmental analysis. FDA issued for public comment a notice of proposed rulemaking on May 1, 1996 (61 FR 19476), that proposed additional categorical exclusions for those actions that have been identified as normally not having a significant impact, individually or cumulatively, on the quality of the human environment. The final rule was published on July 29, 1997 (62 FR 40569) and became effective August 28, 1997.
All applications or petitions requesting Agency action must be accompanied by either an EA or a claim of categorical exclusion. Failure to submit one or the other is sufficient grounds for refusing to file or approve the application (21 CFR 314.101(d)(4), 601.2(a) and (c), and 25.15(a)). An EA that is adequate for filing is one that addresses the relevant environmental issues. An EA adequate for approval is one that contains sufficient information to enable the Agency to determine whether the proposed action may significantly impact the quality of the human environment. Note that an EA document can be submitted in advance of and separate from the main body of the application.
Categorical exclusions for human drugs and biologics are found at 21CFR 25.31. Note that the FDA will require at least an EA for any specific action that ordinarily would be excluded if “extraordinary circumstances” indicate that the specific proposed action may significantly affect the quality of the environment (see below).
CDER’s Guidance for Industry: Environmental Assessment of Human Drug and Biologics Applications (Issued July 1998) provides detailed information on a variety of topics related to preparing and filing EAs. This guidance is now supplemented by Environmental Assessment: Questions and Answers Regarding Drugs With Estrogenic, Androgenic, or Thyroid Activity (Issued March 2016). Additional updates to the 1998 guidance are under consideration. For questions on these updates, see below for contact information.
Categorical Exclusions (21 CFR 25.31)
The following categorical exclusions are among those available for human drugs and biologics:
- NDAs, abbreviated applications, applications for marketing approval of a biologic product, or supplements to such applications or action on an OTC monograph, FDA’s approval of the application does not increase the use of the active moiety (21 CFR 25.31(a));
- NDAs, abbreviated applications, or supplements to such applications if FDA’s approval of the application action on an OTC monograph, increases the use of the active moiety, but the estimated concentration of the substance at the point of entry into the aquatic environment will be below 1 part per billion (ppb) (21 CFR 25.31(b));
- NDAs, abbreviated applications, applications for marketing approval of a biologic product, or supplements to such applications or action on an OTC monograph, for substances that occur naturally in the environment when the approval of the application does not alter significantly the concentration or distribution of the substance, its metabolites, or degradation products in the environment (21 CFR 25.31(c));
- INDs (21 CFR 25.31(e)); and
- applications for marketing approval of a biologic product for transfusable human blood or blood components and plasma (21 CFR 25.31(j)).
To claim a categorical exclusion the applicant must state that the action requested qualifies for a categorical exclusion, citing the particular categorical exclusion that is claimed, and states that to the applicant’s knowledge, no extraordinary circumstances exist (see 21 CFR 25.15; also see Extraordinary Circumstances below). To facilitate Center review, when submitting a claim of categorical exclusion, the sponsor should provide information to support the specific requested exclusion (e.g., calculation of estimated environmental concentrations) and provide supporting data for drugs with estrogenic, androgenic, or thyroid activity (per the Questions and Answers guidance above).
Typically, the following statement is provided:
- *Applicant’s name *claims that approval of this (A)NDA qualifies for a categorical exclusion in accordance with 21 CFR 25.31(x) and that, to the best of the applicant’s knowledge, no extraordinary circumstances exist which may significantly affect the quality of the human environment. The following information is provided to support this exclusion: …
Drug or Biological Products Derived from Plants or Animals
As described in Environmental Assessment of Human Drug and Biologics Applications, for actions where the drug or biological product is derived from plants or animals, CDER requests that the applicant provide the following information with the claim, or specifically identify where the information can be located (e.g., page number of application):
- biological identification (i.e., common names, synonyms, variety, species, genus and family);
- a statement as to whether wild or cultivated specimens are used;
- the geographic region (e.g., country, state, province) where the biomass is obtained; and
- a statement indicating: (a) whether the species is determined under the Endangered Species Act or the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) to be endangered or threatened, (b) whether the species is entitled to special protection under some other Federal law or international treaty to which the United States is a party, (c) whether the species is the critical habitat of a species that has been determined to be endangered or threatened under the Endangered Species Act or CITES, (d) whether the species is the critical habitat of a species entitled to special protection under some other Federal law or international treaty to which the United States is a party.
Extraordinary Circumstances (21 CFR 25.21)
As required under 40 CFR 1508.4, FDA will require at least an EA for any specific action that ordinarily would be excluded if extraordinary circumstances indicate that the specific proposed action may significantly affect the quality of the human environment (see 40 CFR 1508.27 for examples of significant impacts). Examples of such extraordinary circumstances include:
- Actions for which available data establish that, at the expected level of exposure, there is the potential for serious harm to the environment; and
- Actions that adversely affect a species or the critical habitat of a species that are entitled to special protection as determined under the Endangered Species Act or the Convention on International Trade in Endangered Species of Wild Flora and Fauna or some other Federal law.
Contact:
Please email the CDER EA Team with any questions, at: CDER.EA.Team@fda.hhs.gov.